Cuban Advanced Cancer Vaccines, Novel Medications and Drugs
Breaking News
Novel drug Itolizumab monoclonal antibody developed in Cuba for treatment of lymphomas and leukemia now being used with COVID-19 patients
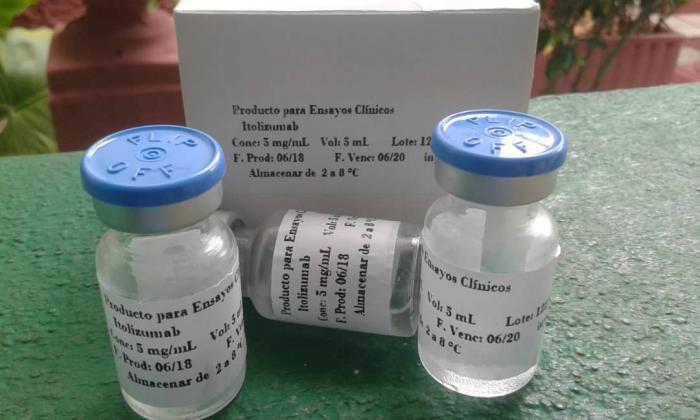 In 2014 the novel medication won a National Award from the Cuban Academy of Sciences (ACC) in the Biomedical Sciences arena, and since April, this year, the humanized monoclonal antibody, Itolizumab, has been included in the country's COVID-19 treatment protocol.
In 2014 the novel medication won a National Award from the Cuban Academy of Sciences (ACC) in the Biomedical Sciences arena, and since April, this year, the humanized monoclonal antibody, Itolizumab, has been included in the country's COVID-19 treatment protocol.
Itolizumab is a molecule that was developed for treatment of lymphomas and leukemia. This antibody is able to block the proliferation and activation of t-lymphocytes, acting as an immunomodulator. As part of its active mechanisms, it can reduce the secretion of a group of inflammation mediators, known as pro-inflammatory cytokines. In Cuba it had been used successfully in the treatment of patients with rheumatoid arthritis and psoriasis. Precisely, in clinical trials carried out in these autoimmune diseases, the monoclonal proved to be a very safe molecule, not causing adverse side effects in patients.
What is a cytokine storm? According to various studies conducted around the world, a group of COVID patients develop an overactive immune reaction. To explain this in a simple way, after the elevated secretion of pro-inflammatory cytokines, blood vessels dilate to allow the immune cells to enter tissues, where viral replication must be reduced. In some patients, a large outflow of substances and fluid occurs in the lungs and blood pressure drops. In order to stop the massive outflow of these substances, a coagulation cascade is activated, resulting in the obstruction of blood vessels in the lungs. This causes the patient great difficulty in gas exchange and hypoxia ensues. As pressure inside the lungs increases, heart failure can occur. Unfortunately, many patients die as a result of cardio-respiratory complications. If other organs do not receive enough oxygen, permanent damage or even death may follow. The monoclonal antibody Itolizumab acts in the disease phase, when damage is caused by the immune system's exaggerated response to the virus' enormous ability to divide. Thus, Itolizumab manages to reduce the secretion of these inflammatory cytokines, which cause the massive flow of substances and liquid in the lungs.
What preliminary results have been obtained? The monoclonal antibody has been used as part of an expanded protocol for COVID-19. It was also approved by the Ethics Committee and by the Cuban regulatory agency, the State Center for the Control of Drugs, Equipment and Medical Devices (Cecmed). Thus far, more than 70 patients with the virus have been treated, in nine hospitals in Cuba. In particular, it has been used with patients in critical, serious and cautionary condition, at high risk for further complications. The best results have been seen in critically ill and cautionary patients, where the consequences of the cytokine storm are stopped in time. Likewise, in many cases there is clinical and imaging evidence of improvement in respiratory distress.
Cuban Interferon alfa 2B used in China against Coronavirus
 Cuban medication "Interferon alfa 2B" is among about 30 drugs chosen by the Chinese National Health Commission. Chinese-Cuban pharmaceutical plant in Jilin (China) has been producing Interferon alpha (IFNrec) from the first day of the Lunar New Year with the use of Cuban technology. The Health Commission of China has selected Cuban product among those used in the fight against coronavirus 2019-NCOV. According to specialists, Cuban IFNrec is applied against viral infections caused by HIV, recurrent respiratory papillomatosis caused by human papillomavirus, accumulated condyloma and hepatitis types B and C, in addition to being effective in therapies against different types of cancer.
Cuban medication "Interferon alfa 2B" is among about 30 drugs chosen by the Chinese National Health Commission. Chinese-Cuban pharmaceutical plant in Jilin (China) has been producing Interferon alpha (IFNrec) from the first day of the Lunar New Year with the use of Cuban technology. The Health Commission of China has selected Cuban product among those used in the fight against coronavirus 2019-NCOV. According to specialists, Cuban IFNrec is applied against viral infections caused by HIV, recurrent respiratory papillomatosis caused by human papillomavirus, accumulated condyloma and hepatitis types B and C, in addition to being effective in therapies against different types of cancer.
Biomodulina T Cuban medication to treat COVID-19 in older adults
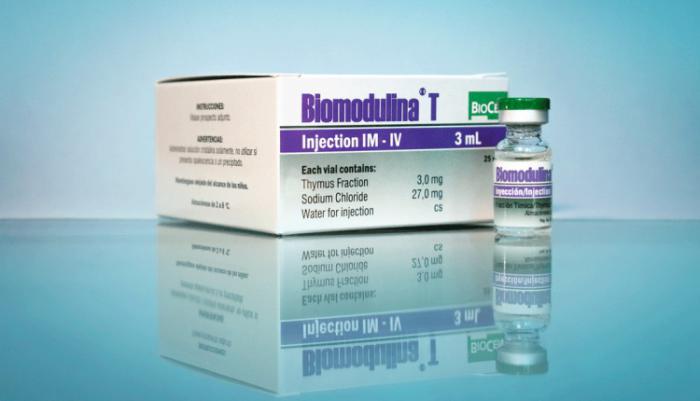 Biomodulina T does not prevent contagion with the new coronavirus, but rather helps stimulate the immune systems of individuals in vulnerable groups, to improve their response in case of infection, and reduce complications. Cuba has confirmed its status as a leader among suppliers of drugs needed to fight the new coronavirus, responsible for all production on the island of the key medication Recombinant Human Interferon Alpha 2b. Another important product, unique to the center, Biomodulina T is manufactured on a large scale at the center's plants, as well. the latter is an immunomodulator made from natural products, which has proven effectiveness in the treatment of respiratory diseases in older adults, which led to its inclusion on the Ministry of Public Health's list of 22 medicines to be used to treat COVID-19 in Cuba. The drug serves to support the immune system of patients and is used as a preventative for staff working directly with confirmed and suspected cases. Cuban health authorities have clarified that Biomodulina, used successfully in Cuba for over 12 years, does not prevent infection, but rather helps stimulate the immune systems of individuals in vulnerable groups, to improve their response to viruses in case of infection, and reduce complications. Biomodulina T is a biological immunomodulator, of totally natural origin, composed of specific extracts from bovine thymus. Its principal effect is to stimulate the production of T-lymphocytes and strengthen the differentiation of the lymphoblastoid cells in the thymus, one of the immune system's most important glands. The thymus plays a vital role in the immune system, producing and secreting an array of polypeptides and hormones that act on T-cell differentiation, ensuring the normal development of thymus-dependent cellular and humoral immunity mechanisms, and particularly, the maturation and differentiation of T-lymphocytes. This injectable medication has been registered to treat cellular-type immune dysfunction, including respiratory infections in the elderly. The efficacy and safety of its use to combat these conditions in this population group is supported by its medical indication and marketing for several years, with minimal adverse side effects observed. The pharmacological effect and safety of Biomodulina T have been demonstrated in animal models and clinical trials in humans. The product currently has clinical indication for the treatment of recurrent respiratory infections in geriatric patients, given its immune-restorative effect. According to the product's research report, Biomodulina T has been shown to be useful in slowing the process of immunosenescence, or gradual deterioration of the immune system caused by natural aging.
It is important to note that even when the function of the thymus decreases with age, the maturing function of T-cells is not limited to the thymus gland alone and can be found in other lymphoid tissues as well, while thymic hormones can have a systemic effect. Several pilot clinical studies, suggest its potential efficacy in various immune pathologies, particularly cellular immune deficiencies in childhood, in patients with HIV/AIDS, autoimmune diseases, allergy and as a complementary treatment for cancer patients undergoing radiation and chemotherapy. In the face of COVID-19 global pandemic, Biocen has proposed including Biomodulina T in the COVID-19 treatment protocol from two perspectives. First, while respecting ethical protocols for clinical trials, use of the drug was proposed in confirmed cases of SARS COV-2 infection in the early stages, as it has been scientifically proven that the disease reduces T lymphocytes. Secondly, the drug was proposed for preventive use in at-risk groups, i.e. older adults and individuals with chronic diseases, including diabetes mellitus or cardiovascular pathologies. Biomodulina T has been included in Cuba's basic drug list for years, with very favorable results in preventing recurrent infections in the elderly. Both clinical experience and knowledge of its active mechanisms suggest that it can be successfully used in treating other immunopathologies. This project is directed toward conducting clinical trials with Cuban patients and abroad in cases of the following immunopathologies: thymic hypoplasia in children, complementary treatment to antiretroviral therapy in HIV/AIDS, severe sepsis, immunorestative therapy in oncological patients undergoing chemo or radiotherapy.
According to the specialists, some 20,000 patients are currently being treated, on the basis of Biocen's current productive capacity. It is estimated that with the planned increase in production levels, approximately 100,000 individuals in Cuba could benefit from Biomodulina T, which would provide coverage for all COVID-19 at-risk groups within the country.
Biomodulina T does not prevent contagion with the new coronavirus, but rather helps stimulate the immune systems of individuals in vulnerable groups, to improve their response in case of infection, and reduce complications. Cuba has confirmed its status as a leader among suppliers of drugs needed to fight the new coronavirus, responsible for all production on the island of the key medication Recombinant Human Interferon Alpha 2b. Another important product, unique to the center, Biomodulina T is manufactured on a large scale at the center's plants, as well. the latter is an immunomodulator made from natural products, which has proven effectiveness in the treatment of respiratory diseases in older adults, which led to its inclusion on the Ministry of Public Health's list of 22 medicines to be used to treat COVID-19 in Cuba. The drug serves to support the immune system of patients and is used as a preventative for staff working directly with confirmed and suspected cases. Cuban health authorities have clarified that Biomodulina, used successfully in Cuba for over 12 years, does not prevent infection, but rather helps stimulate the immune systems of individuals in vulnerable groups, to improve their response to viruses in case of infection, and reduce complications. Biomodulina T is a biological immunomodulator, of totally natural origin, composed of specific extracts from bovine thymus. Its principal effect is to stimulate the production of T-lymphocytes and strengthen the differentiation of the lymphoblastoid cells in the thymus, one of the immune system's most important glands. The thymus plays a vital role in the immune system, producing and secreting an array of polypeptides and hormones that act on T-cell differentiation, ensuring the normal development of thymus-dependent cellular and humoral immunity mechanisms, and particularly, the maturation and differentiation of T-lymphocytes. This injectable medication has been registered to treat cellular-type immune dysfunction, including respiratory infections in the elderly. The efficacy and safety of its use to combat these conditions in this population group is supported by its medical indication and marketing for several years, with minimal adverse side effects observed. The pharmacological effect and safety of Biomodulina T have been demonstrated in animal models and clinical trials in humans. The product currently has clinical indication for the treatment of recurrent respiratory infections in geriatric patients, given its immune-restorative effect. According to the product's research report, Biomodulina T has been shown to be useful in slowing the process of immunosenescence, or gradual deterioration of the immune system caused by natural aging.
It is important to note that even when the function of the thymus decreases with age, the maturing function of T-cells is not limited to the thymus gland alone and can be found in other lymphoid tissues as well, while thymic hormones can have a systemic effect. Several pilot clinical studies, suggest its potential efficacy in various immune pathologies, particularly cellular immune deficiencies in childhood, in patients with HIV/AIDS, autoimmune diseases, allergy and as a complementary treatment for cancer patients undergoing radiation and chemotherapy. In the face of COVID-19 global pandemic, Biocen has proposed including Biomodulina T in the COVID-19 treatment protocol from two perspectives. First, while respecting ethical protocols for clinical trials, use of the drug was proposed in confirmed cases of SARS COV-2 infection in the early stages, as it has been scientifically proven that the disease reduces T lymphocytes. Secondly, the drug was proposed for preventive use in at-risk groups, i.e. older adults and individuals with chronic diseases, including diabetes mellitus or cardiovascular pathologies. Biomodulina T has been included in Cuba's basic drug list for years, with very favorable results in preventing recurrent infections in the elderly. Both clinical experience and knowledge of its active mechanisms suggest that it can be successfully used in treating other immunopathologies. This project is directed toward conducting clinical trials with Cuban patients and abroad in cases of the following immunopathologies: thymic hypoplasia in children, complementary treatment to antiretroviral therapy in HIV/AIDS, severe sepsis, immunorestative therapy in oncological patients undergoing chemo or radiotherapy.
According to the specialists, some 20,000 patients are currently being treated, on the basis of Biocen's current productive capacity. It is estimated that with the planned increase in production levels, approximately 100,000 individuals in Cuba could benefit from Biomodulina T, which would provide coverage for all COVID-19 at-risk groups within the country.
Medical News from Cuba
Clinical trials of Cuban lung cancer vaccine in the U.S. to be continued
The Roswell Park Comprehensive Cancer Center announced on Wednesday that the initial results of the first North American clinical study of the CIMAvax-EGF vaccine show that this immunotherapy developed in Cuba is safe, well tolerated and deserves further study.
Racotumomab (VAXIRA). World's first lung cancer vaccine
Cuba patents second therapeutic vaccine for non-small cells lung cancer. While US global healthcare groups still working on a similar one
 In 2012, Cuba patented the first therapeutic vaccine in the world against advanced lung cancer, called CIMAVAX-EGF. In January 2013, the island announced the second cancer vaccine, known as Racotumomab.
In 2012, Cuba patented the first therapeutic vaccine in the world against advanced lung cancer, called CIMAVAX-EGF. In January 2013, the island announced the second cancer vaccine, known as Racotumomab.
Clinical tests, carried out in 86 nations, revealed that though these vaccines do not cure the disease, they do reduce the tumors thus improving the quality and expectancy of life of the patients.
Cuban scientists have developed the world's first lung cancer vaccine, that clinical trials have shown prolonged the life of three times more patients in the late stages of the disease than traditional chemotherapy and radiotherapy treatments.
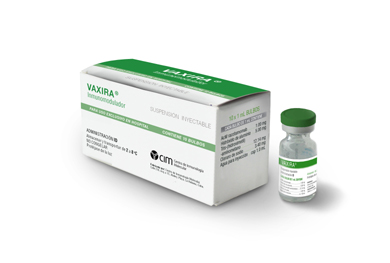 The medication, Racotumomab, is not a vaccine in the ordinary sense. It neither prevents, nor cures, what is one of the most aggressive and hard-to-heal cancers, and one which is usually detected only when it is already very advanced. The drug offers a novel and effective way of boosting treatment of lung cancer
and is hoped to be selling in 25 countries by 2015.
The medication, Racotumomab, is not a vaccine in the ordinary sense. It neither prevents, nor cures, what is one of the most aggressive and hard-to-heal cancers, and one which is usually detected only when it is already very advanced. The drug offers a novel and effective way of boosting treatment of lung cancer
and is hoped to be selling in 25 countries by 2015.
Of course, this is not a miracle cure. But it does provide an option so that, in combination with chemotherapy and radiotherapy, it can prolong survival significantly.
Clinical trials on some 1,700 patients have shown that 24 per cent of late-stage lung cancer sufferers lived for two years with Racotumomab, whereas the figure was only 8 per cent on chemotherapy and radiotherapy. Phase III trials are continuing in seven countries.
Whereas those traditional treatments kill cancer cells but attack all cells, Racotumomab is one of a new breed of drugs designed to spark the body's immune system into destroying the cancer by "unmasking" it and allowing the body to kill it.
This is an interesting approach to tackling a very hard-to-treat cancer, where new therapies are urgently needed.
The research and early clinical trials so far look encouraging for this experimental new treatment vaccine, although the initial results indicate the drug extends survival rather than curing the disease.
The researchers have found it to be most effective in patients whose tumours have been shrunk, but not eliminated, after chemotherapy or radiotherapy, and they have also tested it on people who cannot tolerate such an aggressive traditional treatment regime. But for now, at least, it is not seen as a first port of call for lung cancer sufferers.
Patients receive the vaccine over the course of a year – five individual doses, each two weeks apart, and then a maintenance dose every month, with the possibility of the same brief flu-like side-effects associated with many inoculations.
Not all lung cancers are the same, but Racotumomab can be used in the so-called Non-Small Cell lung cancers that account for about 85 per cent of cases and probably some lung mesotheliomas. With average cost per patient around $20,000.
Racotumomab is currently available in Cuba and it is expected to go on sale in Asia as well as Latin America, and the team is working on approval in Europe. However, the US is a "problem", because the Office of Foreign Assets Control needs to give authorisation because of the US embargo against Cuba.
The scientists believe the drug, or ones like it, could eventually have other applications – breast cancer, for example, can have the same tumour antigen as lung cancer.
Superior Efficacy and Safety of a Nonemulsive Variant of the VSSP Vaccine in Advanced Breast Cancer Patients
Very small-sized proteoliposomes (VSSP) is another promising cancer drug invented by Havana Center of Molecular Immunology (CIM). VSSP was originally designed as a compound to help boost the immune response to vaccines, but also appears to enhance the anti-cancer immune response. This drug isn't as fully developed as CimaVax, but appears to have great potential.
VSSP vaccine consists in VSSP obtained by the incorporation of NGcGM3 into the outer membrane protein complex of N. meningitidis. In Cuba VSSP has been studied in patients with advanced breast cancer as an emulsive formulation. Recently, this vaccine has also been tested as a nonemulsive formulation (without Montanide ISA 51).
USA Roswell Park Cancer Institute is preparing VSSP for three trials — two in kidney cancer and one in breast cancer.
Novel skin cancer medication "Heberferon" now available nation-wide
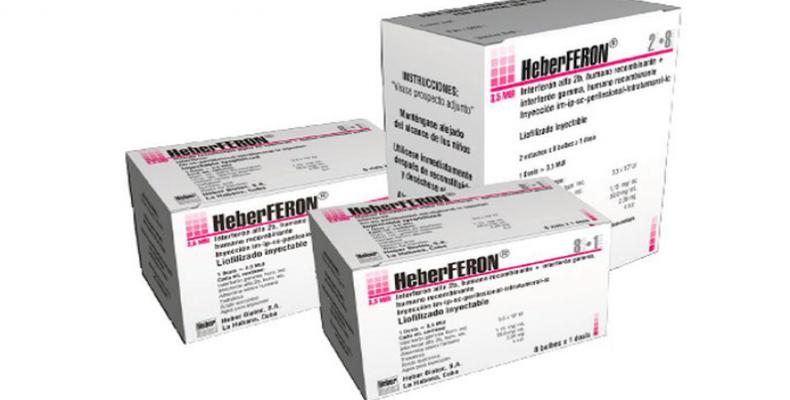 After 20 years of research and clinical trials, Cuba developed new one-of-a-kind medication to treat skin cancer (basal cell carcinoma) called "Heberferon" is now available nation-wide. The novel drug which combines interferon "Alfa" and interferon "Gamma" through the use of recombinant technology is likely to be unique in the world. HeberFERON is prescribed for perilesional (intradermal) or intralesional therapies of basal cell carcinoma previously confirmed by a biopsy. The drug can be used as alternative or adjuvant therapy with other treatments (surgical or not), in cases of lesions of any size or clinical subtype and in any high-risk area (H mask area of face) or locally advanced lesions that are difficult to treat due to local invasion and/or proximity to vital structures such as the eyes and brain. The injectable medication cures or reduces the size of malignant skin tumors and can prevent scaring from surgery in areas difficult to operate on such as the face. Cuba has a high rate of skin cancer, which is usually caused by excessive exposure to the sun. Havana-based production plants have already manufactured over 10,000 vials of the new medicine, which continues to be developed with the aim of treating other types of cancer. Meanwhile the next step will be to include Heberferon on the island's list of basic medicines.
After 20 years of research and clinical trials, Cuba developed new one-of-a-kind medication to treat skin cancer (basal cell carcinoma) called "Heberferon" is now available nation-wide. The novel drug which combines interferon "Alfa" and interferon "Gamma" through the use of recombinant technology is likely to be unique in the world. HeberFERON is prescribed for perilesional (intradermal) or intralesional therapies of basal cell carcinoma previously confirmed by a biopsy. The drug can be used as alternative or adjuvant therapy with other treatments (surgical or not), in cases of lesions of any size or clinical subtype and in any high-risk area (H mask area of face) or locally advanced lesions that are difficult to treat due to local invasion and/or proximity to vital structures such as the eyes and brain. The injectable medication cures or reduces the size of malignant skin tumors and can prevent scaring from surgery in areas difficult to operate on such as the face. Cuba has a high rate of skin cancer, which is usually caused by excessive exposure to the sun. Havana-based production plants have already manufactured over 10,000 vials of the new medicine, which continues to be developed with the aim of treating other types of cancer. Meanwhile the next step will be to include Heberferon on the island's list of basic medicines.
Hebervital notable benefits in different pathologies
 Developed in Cuba, Hebervital is used in patients receiving chemotherapy, radiation therapy, bone marrow transplants and immune-depressed persons, experts say. It is also applied to patients with decreased body defenses and with a history of severe or recurrent infections, and its benefits includes reduction of antibiotic use and frequency of hospitalization.
Developed in Cuba, Hebervital is used in patients receiving chemotherapy, radiation therapy, bone marrow transplants and immune-depressed persons, experts say. It is also applied to patients with decreased body defenses and with a history of severe or recurrent infections, and its benefits includes reduction of antibiotic use and frequency of hospitalization.
The timely use of this drug allows completing the chemotherapy and radiation therapy regimen in cancer patients, which provides better treatment effectiveness and quality of life. According to experts, Hebervital is a first-line stimulant factor in supporting cancer therapy because it increases the number of stem cells, which are subsequently collected to be infiltrated. It has shown effectiveness in hepatic cirrhosis, peripheral arterial insufficiency, pathologies of the knee and hip joints, as well as in skin lesions, alopecia and scars due to juvenile acne, among other ailments.
Cuban HIV vaccine´s clinical trial is underway
 A therapeutic vaccine aimed at reducing the viral load of patients with Human Immunodeficiency Virus (HIV), which has an impact on the quality of life of patients, is currently in phase one of the clinical trial, in which its safety is studied. Nine patients who the vaccine was tested in didn't show any adverse effects or toxicity, which is the main objective of this phase. Its administration is carried out simultaneously through the mucosal route, with the use of spray and intramuscular vaccination, and it has been preliminarily verified that it diminishes the viral load in the CD8 cells.
A therapeutic vaccine aimed at reducing the viral load of patients with Human Immunodeficiency Virus (HIV), which has an impact on the quality of life of patients, is currently in phase one of the clinical trial, in which its safety is studied. Nine patients who the vaccine was tested in didn't show any adverse effects or toxicity, which is the main objective of this phase. Its administration is carried out simultaneously through the mucosal route, with the use of spray and intramuscular vaccination, and it has been preliminarily verified that it diminishes the viral load in the CD8 cells.
After preclinical studies in laboratory animals, and tests in the small group of humans, it was shown that the immune response of the organism is enhanced thanks to the use of TERAVAC-VIH, although scientists insist on not creating false expectations. This is a multi-year project, and will take time which will include testing phases with a greater number of seropositives in which large-scale and comprehensive efficacy will be tested to determine whether or not to continue with its use. What is important is that Cuban scientific institutions keep the search for vaccine candidates against HIV among its research priorities, although prevention is still the main method of avoiding contagion.
The goal is to replace the current tripartite therapy, combining several methods that prevent the development of HIV, highly effective because the retroviral inhibitors block the spread of the virus, but can cause collateral damage and force in some cases the suspension of the treatment for a time.
New chronic hepatitis B medication registered in Cuba
Cuba's HeberNasvac® vaccine, unique in the world for treating patients infected by the virus of chronic Hepatitis B, obtained the health registration by the Center for State Control of Drugs, Equipment Medical Devices. Developed by the Center for Genetic Engineering and Biotechnology, the therapeutic vaccine is supported by patents granted in the most demanding markets, and has more than 20 scientific publications by Cuban researchers with foreign contributors. In addition to the clinical study in Bangladesh, which demonstrated the validity of the vaccine and allowed the granting of registration, clinical research continues, for which a study on efficacy is performed in conjunction with the French counterpart, Abivax, that includes 280 patients from eight Asian countries and develops successfully in this region of the world, among the hardest hit by the virus. The research has been approved by regulatory authorities of Australia, New Zealand, Singapore, South Korea, Taiwan, Hong Kong, Philippines and Thailand and the clinical protocol was designed by international experts of the highest scientific level, in cooperation with Cuban specialists.
Administered through the nose or subcutaneously, the promising product features improved antiviral properties with fewer adverse effects than other similar products used worldwide.
Cuba begins 3rd phase of clinical trials of prostate cancer vaccine
Cuban scientists are drafting the protocols for the third phase of clinical trials of a potential treatment for advanced prostate cancer.
The therapy, named Heberprovac, was developed at the Genetic Engineering and Biotechnology Center, or CIGB, in the east-central province of Camaguey.
After the second phase of the trial was completed in early 2015, Heberprovac was approved for "safety and efficacy" by Cuba's State Bureau of Medications, Medical Equipment and Devices.
The next step in the clinical trial is to compare the efficacy of the new Cuban medicine with Zoladex, an internationally known therapy for several types of cancer.
The hope is that the Cuban compound will be able to compete with Zoladex in resistance to hormonal castration, either delaying its appearance or reverting it. Prostate adenocarcinoma is a tumor feeding on testosterone, and both therapies inhibit the production of the male hormone, thus reducing the afflictions associated with this type of cancer.
Heberprovac's administration schedule, which includes seven injections - the first four every 14 days and the rest once a month - is better than Zoladex's schedule, CIGB scientists said.
Zoladex, which is also used to treat breast cancer, is administered every three months via subcutaneous implant.
Although Heberprovac cannot be described yet as a cure for prostate cancer, most of the patients treated show longer survival rates and a better quality of life.
CimaVax EGF can turn advanced lung cancer into a manageable chronic illness
CIMAVAX EGF eligibility, model program and average cost of treatment please see here
 Pretty big news comes from Cuba. Cuban medical authorities have released the first therapeutic vaccine for lung cancer. CimaVax-EGF is the result of a 25-year research project at Havana's Center for Molecular Immunology, and it could make a life or death difference for those facing late-stage lung cancers.
Pretty big news comes from Cuba. Cuban medical authorities have released the first therapeutic vaccine for lung cancer. CimaVax-EGF is the result of a 25-year research project at Havana's Center for Molecular Immunology, and it could make a life or death difference for those facing late-stage lung cancers.
CimaVax-EGF isn't a vaccine in the preventative sense--that is, it doesn't prevent lung cancer from taking hold in new patients. It's based on a protein related to uncontrolled cell proliferation--that is, it doesn't prevent cancer from existing in the first place but attacks the mechanism by which it does harm. As such it can turn aggressive later-stage non-small cells lung cancer into a manageable chronic disease by creating antibodies that do battle with the proteins that cause uncontrolled cell proliferation. Chemotherapy and radiotherapy are still recommended as a primary means of destroying cancerous tissue, but for those showing no improvement the new vaccine could be a literal lifesaver.
The vaccine has already been tested in 1,000 patients in Cuba and is being distributed at hospitals there free of charge. Cimavax has proven to be one of the most effective ways to treat lung cancer that is in the late stages (stage IIIB to IV). The vaccine works by targeting the protein called epidermal growth factor (EGF).
Many cancers will force the body to produce large amounts of EGF which makes the cells grow and divide and unable to control. Cimavax is partially composed of EGF and tells the body to create antibodies which identify and bind to the EGF stopping it from being able to attach to the receptors of the cancer cells. By doing this, Cimavax will cut off the signal that the cancer sends to its cell to grow and divide which in return will slow down the growth of the lung cancer. The Cimavax vaccine does not prevent lung cancer, but it can change your condition from life threatening to a chronic and manageable condition.
 Havana-based Centre of Molecular Immunology has developed the anti-cancer drug nimotuzumab, a humanized monoclonal antibody that recognized the epidermal growth factor receptor with a high affinity for the сancer treatment to treat advanced tumours, for example in the head, neck and brain. Nimotuzumab is a monoclonal antibody that mimics human immune cells and binds to specific target molecules of cancer cells. It targets a protein that can cause uncontrolled cell division and growth. The medicine is currently going through clinical trials in Japan and Europe.
Havana-based Centre of Molecular Immunology has developed the anti-cancer drug nimotuzumab, a humanized monoclonal antibody that recognized the epidermal growth factor receptor with a high affinity for the сancer treatment to treat advanced tumours, for example in the head, neck and brain. Nimotuzumab is a monoclonal antibody that mimics human immune cells and binds to specific target molecules of cancer cells. It targets a protein that can cause uncontrolled cell division and growth. The medicine is currently going through clinical trials in Japan and Europe.
Daiichi Sankyo Company Ltd of Tokyo, Japan, has announced that it has enrolled the first patient to begin Phase III clinical trial with nimotuzumab (DE-766) in patients with lung cancer and stomach cancer.
Heberprot-p: intralesional infiltrations can prevent amputations in patients with advanced diabetic foot wounds
 One of the problems associated with diabetes is diabetic foot ulcer, an extremely serious condition with a very high incidence among patients. In fact, according to the U.S. Center for Disease Control and Prevention, up to 15 percent of diabetics will develop that condition. Just 90 miles away, Cuba's Institute of Genetic Engineering and Biotechnology has developed a new medical product, Heberprot-P, which can halt, and in some cases, cure, diabetic foot ulcers. This medicine, unique in the world, uses another achievement of Cuban medicine, recombinant human epidermal growth factor (rhEGF), and it brings relief and prevents the amputation of the limbs affected. The effectiveness of this medicine is backed by its sanitary registration in 20-some countries and patents granted in 40 countries. Powerhouses such as Brazil and China are hoping to obtain the necessary license to use this medication, and in Europe, the product's pre-clinical stage has ended, with good toxicology and safety results. However, in the United States, the number three country affected by diabetes (following China and India), this new Cuban medicine that would improve the quality of life of millions of patients cannot be sold. The laws of the embargo prohibit it, unless the administration issues special measures and licenses for the necessary tests to be carried out, and for a large population of patients to benefit.
One of the problems associated with diabetes is diabetic foot ulcer, an extremely serious condition with a very high incidence among patients. In fact, according to the U.S. Center for Disease Control and Prevention, up to 15 percent of diabetics will develop that condition. Just 90 miles away, Cuba's Institute of Genetic Engineering and Biotechnology has developed a new medical product, Heberprot-P, which can halt, and in some cases, cure, diabetic foot ulcers. This medicine, unique in the world, uses another achievement of Cuban medicine, recombinant human epidermal growth factor (rhEGF), and it brings relief and prevents the amputation of the limbs affected. The effectiveness of this medicine is backed by its sanitary registration in 20-some countries and patents granted in 40 countries. Powerhouses such as Brazil and China are hoping to obtain the necessary license to use this medication, and in Europe, the product's pre-clinical stage has ended, with good toxicology and safety results. However, in the United States, the number three country affected by diabetes (following China and India), this new Cuban medicine that would improve the quality of life of millions of patients cannot be sold. The laws of the embargo prohibit it, unless the administration issues special measures and licenses for the necessary tests to be carried out, and for a large population of patients to benefit.
Heberprot-P is and injectable formula that steps up the cicatrization of deep and complex ulcers, thus reducing the number of amputations. Heberprot-P, a medication produced by Cuban biotechnology, reduces the number of surgical operations, cicatrization time, possible complications like gangrene and infections, and avoids high costs derived from long-time hospitalization; it also improves quality of life and the functional recovery of patients. 85 percent of amputations can be prevented, while 70 percent of those surgeries are applied on patients suffering from diabetes. It is important to note that the US economic blockade of Cuba hinders the marketing of Cuban pharmaceuticals in the United States. For instance, a total of 80 thousand diabetic people who undergo toe amputation in the United States every year cannot access the Cuban vaccine known as Heberprot-P, which precisely avoids such amputations.
A study examined if a series of epidermal growth factor (EGF) local infiltrations can enhance the healing process of complicated diabetic wounds. Twenty-nine in-hospital patients with diabetic neuropathic or ischaemic lesions with high risk of amputation were treated in a non controlled pilot study conducted at the National Institute of Angiology, Havana. Lesions, classified as Wagner's grade 3 or 4, included ulcers 20 cm2 for 25 days or amputation residual bases 30 cm2 for 15 days, healing refractory despite comprehensive wound care. EGF (25 mg) intralesional infiltrations (250 l of a 25 g/ml solution/injection point) were performed thrice weekly up to the eighth week. Wound closure was monitored during the treatment and recurrence examined for a year following discharge from hospital. Eighty-six per cent of the patients treated showed a productive granulation at infiltration session 8. Histological examination at this point indicated a substantial wound matrix transformation, granulation tissue cell repopulation and angiogenesis. Of the 29 patients treated, amputation was prevented in 17 (58.6%) of them who completed 24 infiltration sessions. They averaged 71.1 ± 18.3% of reepithelisation during a mean in-hospital period of 66.5 ± 4.9 days. Wound recurrence after 1 year of follow-up appeared in only one patient. Preliminary evidences suggest that EGF intralesional infiltrations may be effective in reducing diabetic lower limb amputation.
Cuban drug benefits two thousand patients with Hepatitis C
Over 250 gastroenterologists dedicated to the care of liver diseases in Cuba presented the results of use of PEG-Heberon, Cuban medication for chronic hepatitis C, which from 2010 to date has benefited some two thousand patients.
Alpha-2b Cuban pegylated interferon is marketed under the name PEG-Heberon and presents in dosage form of injectable solution of 1 ml to be administered subcutaneously. The introduction of PEG-Heberon in combination with ribavirin in the treatment of Hepatitis C, has helped to achieve over 40% of SVR in patients with genotype 1 virus without pretreatment. These data are consistent with the reported by existing similar products on the international pharmaceutical market and mean that from a clinical standpoint, 27% and 10% increase in disease control compared to what is achieved with conventional interferon alpha monotherapy and combination ribavirin, respectively. Since 2009, this product has health registration by the Center for State Control of Drugs, Medical Devices, Cuban regulatory agency and has obtained three laurels: the National Health Award in 2013, the Technology Innovation Ministry of Public Health in 2012, and in 2014 the Annual Award of the Academy of Sciences of Cuba. 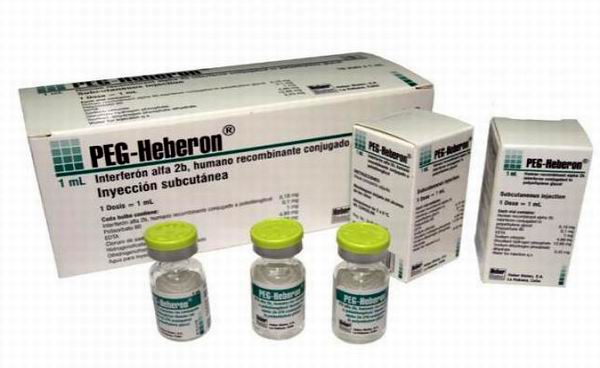 The CIGB is a scientific-production complex with experience in the development of interferon (IFN) alpha since 1981 and possesses the technology necessary to protein pegylation process through which in 2005 he made purification of pegylated conjugate derived from Cuban interferon alfa-2b.
The CIGB is a scientific-production complex with experience in the development of interferon (IFN) alpha since 1981 and possesses the technology necessary to protein pegylation process through which in 2005 he made purification of pegylated conjugate derived from Cuban interferon alfa-2b.
Currently, the association of pegylated interferon and ribavirin is internationally the most accepted therapy for the treatment of chronic hepatitis C.
Vidatox
 The Cuban agenda against cancer is also joined by Labiofam pharmaceutical enterprise, which develops homeopathic medications against the disease, such as VIDATOX, made from the venom of blue scorpion, native of Cuba.
The Cuban agenda against cancer is also joined by Labiofam pharmaceutical enterprise, which develops homeopathic medications against the disease, such as VIDATOX, made from the venom of blue scorpion, native of Cuba.
For more than 20 years, Cubans have been treating cancer patients with blue scorpion venom. And there have been too many success stories to dismiss the miraculous recoveries as coincidence. Even when the results aren't quite as jaw-dropping, thousands attest to pain relief, increased muscle strength, and renewed energy while on the medicine. The treatment is now poised for a global premiere. Cuba's state pharmaceutical company, Labiofam, recently began mass-producing a homeopathic version called Vidatox. A handful of countries have registered it for sale, and a small black market to move the product around the globe has emerged. It's impossible to know how many patients have imbibed the venom treatment from the small glass bottles over the past two decades, but the number is likely more than 55,000 globally.
