Latest Cuba Medical News:
All You Need to Know About Cuba's Lung Cancer Vaccine CIMAVAX-EGF.
Will It Soon Be Available to Everyone in the U.S.?
New heart protector developed in Cuba
 Cuban scientists work on 422 research projects aimed at the prevention and treatment of cancer, cardiovascular and neurodegenerative diseases. One of them called CIGB- 500 and promoted by the Center for Genetic Engineering and Biotechnology is currently in clinical trials stage. This cardio-protective product is able to reduce the size of the infarcted area by 78.9 percent in acute myocardial infarction, which proves its worth considering that cardiovascular diseases are the leading cause of death in Cuba.
Cuban scientists work on 422 research projects aimed at the prevention and treatment of cancer, cardiovascular and neurodegenerative diseases. One of them called CIGB- 500 and promoted by the Center for Genetic Engineering and Biotechnology is currently in clinical trials stage. This cardio-protective product is able to reduce the size of the infarcted area by 78.9 percent in acute myocardial infarction, which proves its worth considering that cardiovascular diseases are the leading cause of death in Cuba.
Heberprot-p unique drug to avoid diabetic amputations soon available in the United Sates
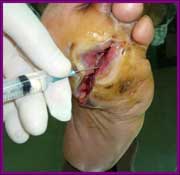 Cuban state run enterprise BioCubaPharma and one of U.S. pharma companies have agreed to work together to make Heberprot-P – a Cuban developed medication to treat diabetic foot ulcers - available to patients in the U.S., once it has been approved by the country's Food and Drug Administration (FDA).
Cuban state run enterprise BioCubaPharma and one of U.S. pharma companies have agreed to work together to make Heberprot-P – a Cuban developed medication to treat diabetic foot ulcers - available to patients in the U.S., once it has been approved by the country's Food and Drug Administration (FDA).
Given the success of the product and following the Memorandum of Understanding signed by the United States Department of Health and Human Services the United States Department of Treasury, Office of Foreign Assets Controls, authorized mentioned U.S. company to enter into agreements with the Cuban party, to conduct all transactions necessary to import Heberprot-P to conduct independent clinical trials authorized by the U.S. Food and Drug Administration (FDA).
Almost one million U.S. citizens are diagnosed with diabetic foot ulcers (DFUs) ever year; a condition which increases the risk of lower-limb amputations. In just five years the number of people suffering from this condition in the U.S. has risen from 73,000 to 85,000, with a significant economic and social impact on the patient, their family members and healthcare system.
Novel neuro-protector to combat Alzheimer's developed in Cuba
 Patients displaying early signs of Alzheimer's will be administered NeuroEpo, a Cuban developed product which has demonstrated neuro-protector qualities in experimental phases. This will be the first time the treatment is used with people and we'll have to wait for the results. The medication doesn't prevent or cure Alzheimer's, however the pre-clinical results are encouraging insomuch as it could help to change the course of the disease, that is to say, it could delay its progression. What Cuban doctors want is to slow down the degenerative process, and improve patients' quality of life.
Patients displaying early signs of Alzheimer's will be administered NeuroEpo, a Cuban developed product which has demonstrated neuro-protector qualities in experimental phases. This will be the first time the treatment is used with people and we'll have to wait for the results. The medication doesn't prevent or cure Alzheimer's, however the pre-clinical results are encouraging insomuch as it could help to change the course of the disease, that is to say, it could delay its progression. What Cuban doctors want is to slow down the degenerative process, and improve patients' quality of life.
People living with dementia and their caregivers have the same right to get respect, inclusion, diagnosis, quality care and treatment. According to international reports, Alzheimer's disease accounts for 50 to 60% of dementia cases in the world; and every three seconds, someone develops this condition and the number of people is expected to double every 20 years, and by 2050 it will total 152 million. Recognized as one of the most significant health crises of the 21st century, dementia is a collective name for progressive brain syndromes that cause the deterioration over time of a variety of different brain functions. They are memory, thought, recognition, language, planning and personality
New skin cancer drug now available nationwide
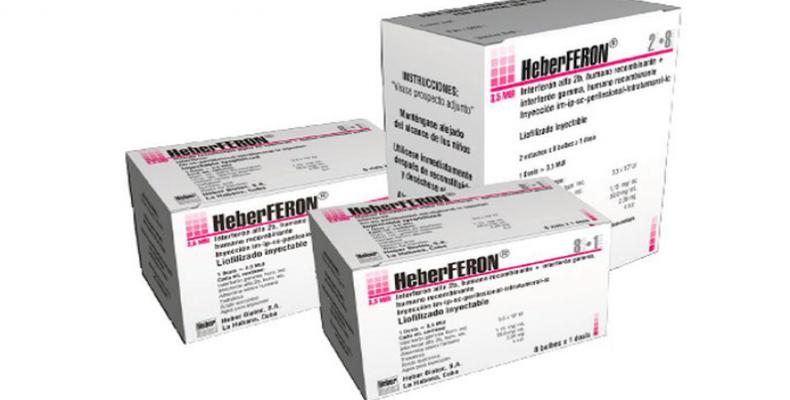 Heberferon, the only Cuban drug of its kind in the world for treatment of basal cell carcinoma of skin, is already available throughout the national territory, barely six months after beginning of the program for its extension. During that period, more than 450 patients in Cuba have benefited from the drug which combines the action of two interferons to inhibit tumor growth and eliminate or reduce lesions, including those that are complex, multiple and in advanced stages or are difficult to surgically access, such as the inner corner of the eye, nasal passages, and others on the face.
Heberferon, the only Cuban drug of its kind in the world for treatment of basal cell carcinoma of skin, is already available throughout the national territory, barely six months after beginning of the program for its extension. During that period, more than 450 patients in Cuba have benefited from the drug which combines the action of two interferons to inhibit tumor growth and eliminate or reduce lesions, including those that are complex, multiple and in advanced stages or are difficult to surgically access, such as the inner corner of the eye, nasal passages, and others on the face.
New Cuban Pneumococcal vaccine to be released next year
 A new and effective Pneumococcal vaccine developed by the Finlay Institute, with conjugates against seven most common serotypes, will be available next year. The vaccine registered under the trademark Quimi-Vio will be available nationwide. Pneumococcal is the main cause of pneumonia and meningitis in infants. Although medicines to protect against this disease are available worldwide, Cuba has not been able to access them due to their high cost. After the effectiveness and safety of the vaccine have been established in extensive preclinical studies, clinical trials on humans began in 2014, where over 5,000 children between one and five years of age have been immunized. Quimi-Vio will therefore be added to Cuba's catalogue of infant vaccines, which already features 10 inoculations against 13 diseases, including diphtheria, mumps, measles, rubella and whooping cough, some of which are produced on the island, such as those against Tuberculosis, Hepatitis B and the pentavalent vaccine.
A new and effective Pneumococcal vaccine developed by the Finlay Institute, with conjugates against seven most common serotypes, will be available next year. The vaccine registered under the trademark Quimi-Vio will be available nationwide. Pneumococcal is the main cause of pneumonia and meningitis in infants. Although medicines to protect against this disease are available worldwide, Cuba has not been able to access them due to their high cost. After the effectiveness and safety of the vaccine have been established in extensive preclinical studies, clinical trials on humans began in 2014, where over 5,000 children between one and five years of age have been immunized. Quimi-Vio will therefore be added to Cuba's catalogue of infant vaccines, which already features 10 inoculations against 13 diseases, including diphtheria, mumps, measles, rubella and whooping cough, some of which are produced on the island, such as those against Tuberculosis, Hepatitis B and the pentavalent vaccine.
Hepatitis Treatment in Cuba. Novel Vaccines and Medications
Cuba Registers Vaccine against Chronic Hepatitis B
Cuba's HeberNasvac® vaccine, unique in the world for treating patients infected by the virus of chronic Hepatitis B, obtained the health registration by the Center for State Control of Drugs, Equipment Medical Devices. This was announced at the 2nd International Congress on Research, Development and Technological Innovation in the Pharmaceutical Industry.
Developed by the Center for Genetic Engineering and Biotechnology (CIGB), the therapeutic vaccine is supported by patents granted in the most demanding markets, and has more than 20 scientific publications by Cuban researchers with foreign contributors. Among them, from the Ehime University in Japan, the Liver Foundation and the Society for the Study of Liver of Bangladesh, the Pasteur Institute of France, the Venezuelan Institute for Scientific Research, and the University of Hanover, of Germany.
Use of stem cells in Cuba encouraging results
 The specialties of Angiology, Orthopedics and Traumatology treasure by this order the largest number of cases with the use of stem cells in Cuba. By the end of 2017, the total number of domestic patients treated with stem cells amounted to 3000. The use of stem cells is promising in therapy of people suffering from serious renal insufficiency of the lower limbs, to avoid amputation in a range of 58 to 80% of patients who had this ailment. A marked improvement is also recorded in about 85% of patients presenting chronic arterial insufficiency of the lower members, who, because of the intense pain, were obliged to stop the walk before the 150 meters. Some of them today walk a kilometer and more without feeling discomfort. Results are also encouraging in complex fractures, bone cysts, aseptic necrosis of the hip, Arthritis injury joint degenerative, particularly of the knee, periodontitis, heart attacks, paraplegia due to injuries of the spine and spinal cord injury, and in children and adolescents with Muscular Dystrophy Duchenne (DMD). In this latest incurable disease of well-booked evolutionary prognosis, which may cause a total physical disability and more long-term failures of the respiratory system and other vital organs, the application of stem cells has shown a considerable recovery of motor functions that were already quite damaged.
The specialties of Angiology, Orthopedics and Traumatology treasure by this order the largest number of cases with the use of stem cells in Cuba. By the end of 2017, the total number of domestic patients treated with stem cells amounted to 3000. The use of stem cells is promising in therapy of people suffering from serious renal insufficiency of the lower limbs, to avoid amputation in a range of 58 to 80% of patients who had this ailment. A marked improvement is also recorded in about 85% of patients presenting chronic arterial insufficiency of the lower members, who, because of the intense pain, were obliged to stop the walk before the 150 meters. Some of them today walk a kilometer and more without feeling discomfort. Results are also encouraging in complex fractures, bone cysts, aseptic necrosis of the hip, Arthritis injury joint degenerative, particularly of the knee, periodontitis, heart attacks, paraplegia due to injuries of the spine and spinal cord injury, and in children and adolescents with Muscular Dystrophy Duchenne (DMD). In this latest incurable disease of well-booked evolutionary prognosis, which may cause a total physical disability and more long-term failures of the respiratory system and other vital organs, the application of stem cells has shown a considerable recovery of motor functions that were already quite damaged.
Cuban drug and alcohol addiction personalized treatment
Cuban drug and alcohol addiction rehabilitation centers provide personalized treatment programs that meet the needs of each. Counselling and substance abuse treatment is also offered. The rehabilitation centers for the treatment of addictions are located in the so called Therapeutic Communities: The Quinqué and El Cocal, located in the east of the country. These centers provide holisticservices to patients from over 15 countries, mainly Latin Americans, because treatment is basically in Spanish language. The paradigm of comprehensive care in drug dependencies is based on the experience of mental health care in Cuba from the 80s and focused through a model of coexistence where stories, emotions, responsibilities and teamwork are shared, with participatory and natural therapeutic techniques. The treatment program is developed in a country where the drug does not flow with the same impunity as in the rest of the world and where social control mechanisms guarantee the result. Treatment programs have at least 97 days duration and are developed under a closed regime therapeutic community, where discipline is very strict and willingness of the patient and his desire to assist in the recovery are essential. The presence of a relative of the patient during the initial two weeks of income is very suitable for insertion into the community and obtaining useful info for treatment. With guidance and attention from an interdisciplinary team of professionals and technicians led by psychiatrists, based on a phased program ensures treatment of non-consumption of toxic substances (drugs, drugs and alcohol), with a high level ERA, from lifestyle modification, and the application of techniques of control of the situation by the patient. The accommodation is in hotel-type rooms with good comfort, air conditioning, satellite TV. The meals are a la carte and varied and adapted to the needs of patients menu. The centers have swimming pools, sports and games areas, abundant green areas, relaxation and music therapy, acupuncture, occupational therapy, cultural and educational activities and outputs stimulus to historical, cultural and recreational attractions in the region. This program is endorsed by the Society of Psychiatry Cuba.
Model Addictions Treatment Program
Evaluation week + One month of treatment
Evaluation Week
7 days of accommodation. it includes food, nursing standard attention and use of the recreational and therapeutic areas.
Initial evaluation and elaboration of Medical Record
Laboratory investigations:
•Hematocrit
•Hemoglobin
•Leucogram with differencial.
•TGP.
•Midstream clean cash.
•Cholesterol.
•Creatinine.
•Glycemic.
•Erythrosedimentation.
•Serology.
•HIV.
•Feces.
Other Investigations:
•Electrocardiogram
•Radiography of thorax
•Radiography of Skull
•Magnetic Resonance or TAC
•Psychological Tests
•Electro Encephalogram with Brain Mapping
Consultation and re-consultations:
•Specialist in Psychology
•Specialist in Internal Medicine
•Specialist in Physical Culture
•Social Worker
Treatments and Medicines:
•family Psychotherapy
•individual Psychotherapy
•group Psychotherapy
•Medicines for the Control of addiction disorders.
Transfer:
•Transfer in/out airport, arrival to the country.
•(Airport - Hotel, Hotel - Airport)
•Air tickets Havana-Holguín-Habana
PRICE................................$2500.00CUC
Antidependency Program
A Month of Treatment
30 days of accommodation in single room. It includes food, nursing standard attention and use of the recreational and therapeutic areas.
Rehabilitation Treatment:
It is conformed on an individual basis according to the clinical, psychological and physical features, bearing in mind also the social environment of origin, family situation, among other.
The activities carried out, in general, are:
•Group Psychotherapy.
•Individual Psychotherapy.
•Musical Therapy.
•Catharsis.
•Cinema Debates.
•Sessions of Acupuncture and Relaxation.
•Physical Rehabilitation (Physical Culture).
•Psycotherapeutic Activities out of the Center.
•Hired Recreational Activities.
Control Medicines for addiction disorders.
Issue of Medical Summaries and Recommendations.
Monthly Price of Rehabilitation Treatment..............................$4500.00 CUC
Combine your hospital stay with recuperation time at Affordable holiday homes, casas and short-term rentals in Cuba + 190 countries
Cuban scientists discover neuro-protective qualities of new molecule JM-20 for treatment of brain ischemia

Cuban researchers have discovered pharmacological evidence of neuro-protective qualities of new molecule called JM-20, opening up promising prospects for the treatment of cerebral ischemia.
The discovery means that next phase of clinical trails on humans can take place. If successful and the neuro-protective qualities of JM-20 are proven, this could lead to the creation of a medication with effective therapeutic properties to treat brain ischemia and its associated effects. The molecule and its derivatives are protected under a 100% national patent.
Cuba performs over 5,100 successful kidney transplants in 4 decades
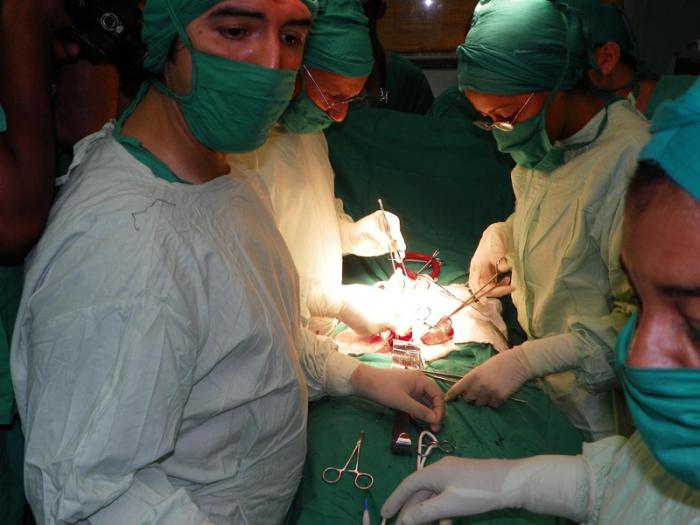 Cuba has successfully performed over 5,100 kidney transplants since 1970, an achievement on par with that of the developed nations. In a 42-year period Cuban surgeons have undertaken 4,751 kidneys transplants with deceased donors and 397 with living donors, all free of charge for Cubans.
Cuba has successfully performed over 5,100 kidney transplants since 1970, an achievement on par with that of the developed nations. In a 42-year period Cuban surgeons have undertaken 4,751 kidneys transplants with deceased donors and 397 with living donors, all free of charge for Cubans.
The living donors are close relatives, such as parents, brothers and sons, of the patients, the only donors Cuban clinics accept for that kind of transplants.
There are 43 hospitals distributed around the country with a multidisciplinary group that attends cadaveric donors with encephalic death. The donor rate in Cuba oscillates between 15 and 18 per million; 90% of them are from cadaveric donors. This program includes 47 dialysis centers throughout the country with 2300 patients as supported by a National Coordinating Center at The Nephrology Institute.
Nine health centers in the country can perform transplants with living donors, five of them in Havana.
Almost 400 liver transplants in Cuba
Cuba has carried out nearly 400 liver transplants since it began this complex proceeding on January 26, 1986, to date. And the results are similar to those of first world nations, despite various limitations on Cuba due to the blockade of United States for over 50 years.
Currently, these grafts are done at two Havana hospitals and William Soler Pediatric Hospital, the latter with a living donor program.
Liver transplantation is a common therapeutics disseminated worldwide to treat patients with acute or chronic liver irreversible illnesses, for which there are no curative treatments. Such transplantation is extremely complex due to the peculiarities of the patient and the surgery that includes within a multidisciplinary group high level anesthetists and surgeons who make dissections and vascular anastomosis necessary to remove the diseased organ and graft the healthy one. According to statistics, in the first world countries the cost of a liver transplant in the first year ranges from 75,000 to 150,000 dollars, and for Cubans is offered for free.
New Cuban Vaccine against Chronic Hepatitis B

New Cuban vaccine for treatment of chronic hepatitis B is currently undergoing clinical trials in Cuba and eight Asian countries, with cooperation of French company Abivax.
The new Cuban medicine called HeberNasvac was developed by researchers of the Center for Genetic Engineering and Biotechnology (CIGB). The new drug that is administered nasally and subcutaneously and has proved to be more effective and safer than all other existing medications available for this virus in the world today.
Studies of clinical evaluation have been approved by regulatory authorities in Australia, New Zealand, South Korea, Singapore, Taiwan, Hong Kong, the Philippines and Thailand.
The protocol for the medical evaluation was designed by leading experts contracted by Abivax along with Cuban scientists, according to officials at the CIGB, who confirmed the satisfactory progress of the trials in Asia, one of the regions most affected by the virus.
A second clinical study of the new vaccine, still in the phase of recruiting the sample of patients, will take place at 13 medical centers in Cuba and will benefit 160 patients on the island.
In Cuba use of the vaccine is expected to begin in 2016, after obtaining the approval of the sanitary registration granted by the Center for State Control of Medicaments, Equipment and Medical Devices (CECMED).
In November 2014, Cuba announced that preventive vaccines against cholera, pneumococcus and hepatitis B were being prepared for registration and application this year. All were described as highly innovative, safe and effective products by medical authorities on the island.
According to the World Health Organization, chronic liver disease caused by the hepatitis B virus is one of the main causes of liver cancer, liver cirrhosis, and other complications such as esophageal varicose veins. Each year there are on the planet about one million deaths related to infection by this virus.
Leading experts from the United States to attend 27th International Congress of Orthopedics and Traumatology in Varadero
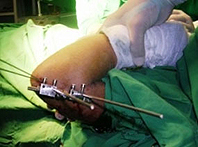 Leading experts from the United States, previously limited from participating due to the laws of the criminal economic, commercial and financial blockade endured by the Cuban people for over 50 years, will offer a symposium on the treatment of shoulder pathologies, where Cuba will present the development of the RALCA external fixator, introduced in surgical procedures on the island. Other key topics of the event include back operations, foot deformities, shoulder, hip and ankle arthroscopy, minimally invasive surgery, infections, complex hip conditions in older adults, injuries in high performance sports (Sports Traumatology) and other developments in the field of orthopedics.More on latest developments in orthopedics and traumatology in Cuba and costs...
Leading experts from the United States, previously limited from participating due to the laws of the criminal economic, commercial and financial blockade endured by the Cuban people for over 50 years, will offer a symposium on the treatment of shoulder pathologies, where Cuba will present the development of the RALCA external fixator, introduced in surgical procedures on the island. Other key topics of the event include back operations, foot deformities, shoulder, hip and ankle arthroscopy, minimally invasive surgery, infections, complex hip conditions in older adults, injuries in high performance sports (Sports Traumatology) and other developments in the field of orthopedics.More on latest developments in orthopedics and traumatology in Cuba and costs...
Malaysian Firm to Commercialize Cuban Vaccine against Cancer in London
Malaysia's biotechnology firm Bioven, which conducts clinical Phase III trials of a promising Cuban vaccine against cancer, plans to list in London after a fruitful cooperation with Cuba. The relationship of Cuba with Bioven, of which the Malaysian government is one of its major shareholders, is an example of South-South cooperation. Since 2008, through an agreement with the Center of Molecular Immunology (CIM) that created the vaccine, Bioven carries out the clinical development of the Cuban vaccine in Europe and part of Asia and Oceania. Since then, Bioven placed the drug, which targets non-small lung cancer cells, in a larger posterior test, and its presence in the Alternative Investment Market, the junior stock market of London, can help speed up commercial thrust. The medication is a kind of immunotherapy that targets proteins called EGF (epidermal growth factor), over expressed by cancer cells. The Phase III trial, the last one before regulatory approval, started in May with the participation of 419 patients from ten countries. Much of the clinical development work is done in Scotland, in cooperation with the Beatson Cancer Institute in Glasgow.
Proctokinasa, safe and effective suppository for the treatment of acute hemorrhoids
 Cuban researchers of the Center for genetic engineering and biotechnology (CIGB), in collaboration with Juan Bruno Zayas Hospital, of Santiago de Cuba, created an effective and safe drug as a suppository for the treatment of acute hemorrhoids, based on the use of recombinant streptokinase that showed its efficacy and safety vs. hydrocortisone acetate-based suppositories in acute hemorrhoidal disease.
Cuban researchers of the Center for genetic engineering and biotechnology (CIGB), in collaboration with Juan Bruno Zayas Hospital, of Santiago de Cuba, created an effective and safe drug as a suppository for the treatment of acute hemorrhoids, based on the use of recombinant streptokinase that showed its efficacy and safety vs. hydrocortisone acetate-based suppositories in acute hemorrhoidal disease.
After more than five years of clinical trials in health institutions of twelve provinces, involving 820 patients, the drug obtained its registration in August 2012 with the name of Proctokinasa and is destined to become the most recommended therapy against acute hemorrhoids in Cuba, depending on growth of productive capacities in nearest future.
Unlike existing local application formulas that focus their main action in reducing inflammation and pain, these suppositories, in addition to meeting the above functions, manage removal of thrombi or clots of blood in the affected anal region, resulting in an improvement of the clinical diagnosis of the hemorrhoid crisis in the range of 75 to 90% of the people treated on the fifth day of employment.
Hemorrhoids are varicose veins or inflammation of the veins of anus. Its key symptom is pain, and they tend to be caused by chronic constipation, diarrheal diagnosis, pregnancy or aging.
Randomized, controlled clinical trials of medical treatments for acute hemorrhoidal disease with recombinant streptokinase suppositories, based on thrombolysis, show a significant efficacy advantage with respect to hydrocortisone acetate, a widely-used, over-the-counter product.
U.S. Mohawk Travel to Cuba for Diabetes Treatment
 The Mohawk tribe urged the U.S. federal government to approve the Cuban diabetes medicine and provide the tribes financial support so they can buy it. A U.S. delegation of the Mohawk tribe of Saint-Régis has traveled to Havana to be treated with Heberprot-P, a Cuban medicine used to treat diabetic foot ulcers. The tribe was receiving treatment for diabetes in Akwesasne, but they decided to be cured by Cuban doctors and to try the effects of Heberprot-P. According to patients, the medicine is still not legally available in the United States, although it is used in more than two dozens nations in the rest of the world. Nevertheless, the medicine is currently being tested in U.S. clinics. "The treatment will be of great help. We have already seen evidence of serious diabetic foot ulcers - of grade 4 and 5 - from which patients have recovered in 45 days," Mohawk said.
The Mohawk tribe urged the U.S. federal government to approve the Cuban diabetes medicine and provide the tribes financial support so they can buy it. A U.S. delegation of the Mohawk tribe of Saint-Régis has traveled to Havana to be treated with Heberprot-P, a Cuban medicine used to treat diabetic foot ulcers. The tribe was receiving treatment for diabetes in Akwesasne, but they decided to be cured by Cuban doctors and to try the effects of Heberprot-P. According to patients, the medicine is still not legally available in the United States, although it is used in more than two dozens nations in the rest of the world. Nevertheless, the medicine is currently being tested in U.S. clinics. "The treatment will be of great help. We have already seen evidence of serious diabetic foot ulcers - of grade 4 and 5 - from which patients have recovered in 45 days," Mohawk said.

Correo Cientifico Medico de Holguin 2006;10(4)
Trabajo original
Hospital Provincial Docente Clinico quirurgico "Lucia Iniguez Landin" Holguin.
Vitiligo en edad pediatrica, Centro de Histoterapia Placentaria Holguin. Cuba. 1999-2004.
Vitiligo at Pediatric Age. Placental Histotherapy Center. Holguin. Cuba. 1999-2004.
Ines Delfina Castilla Huapaya1, Elio Lozano Alvarez2, Onelia M. Hernandez Marrero3, Miriam Bauza Leyva4, Noemi Batista Munoz5.
1 Especialista de primer grado en Dermatologia.
2 Especialista de segundo grado en Endocrinologia. Vicedirector docente del Hospital Lucia Iniguez.
3 Especialista de primer grado en Dermatologia. Profesor Instructor. Jefa de Catedra. Jefa provincial en Dermatologia
4 Especialista de primer grado en Dermatologia. Profesor Asistente. Jefa de Centro Provincial de Histoterapia Placentaria de Holguin.
5 Especialista de primer grado en Dermatologia. Profesor Instructor.
RESUMEN
Se realizo una investigacion de serie de casos en el Centro de Histoterapia Placentaria de Holguin, en el periodo comprendido entre 1999 y 2004 en 186 ninos con el diagnostico clinico e histopatologico de vitiligo, con el objetivo de determinar algunos aspectos clinicos y epidemiologicos de la enfermedad y su respuesta terapeutica a la Melagenina. Predomino el grupo de edad de 10-14 anos con 76 enfermos (40,86%), el sexo femenino con 46 (43,40%) y el tiempo de evolucion fue de 4?6 meses en 70 pacientes (37,63%). Entre los posibles factores desencadenantes ocupo el primer lugar la intranquilidad, presente en 53 enfermos (28,49%). Hubo respuesta favorable al tratamiento con remision parcial de las lesiones en 133 casos (71,51%). Se encontro asociacion con las atopias en 37 pacientes (19,89%). Se concluyo que el vitiligo es una enfermedad frecuente en la edad pediatrica por lo que se les debe dar seguimiento a estos enfermos.
ABSTRACT
An investigation of a serie of cases in the Holguin Center of Placental Histotherapy, in the period between 1999-2004, on 186 children with a diagnosis of histopathologic vitiligo was carried out with the objective of determining some clinical and epidemic aspects of the illness and their therapeutic answer to Melagenina with a predominance of the group 10-14 years with 76 sick persons(40.86%). And 46 females with 43.40%. The time of evolution was from 4 to 6 months in 70 patients(37.63%). Among the possible precipitating factors, uneasiness occupico the first place, with 53 sick persons (28.49%). There was, am favorable response to the treatment with partial remission of the lesion in133 cases (71.50%). Lesions in the trunk occupied the first- place, 59 patients(31.72%). Lesions associated with atrophies were found in 37 patients (19.89%. It was stated that vitiligo is a frequent disease at pediatric age, so there should be a follow-up these patients.
INTRODUCCION
El viiligo es una enfermedad que interesa la piel, de etiologia desconocida, que afecta aproximadamente entre el 1-2 % de la poblacion mundial, independientemente de la edad, sexo y raza. Aproximadamente el 25% son ninos.1 Es una enfermedad relativamente comun, del 2 al 8% de los pacientes dermatologicos la presenta y es mas notorio en las personas de piel oscura. 2
